Cas No: 5949-29-1
EINESC No: 201-069-1
Molecular weight: 192.123 g/mol
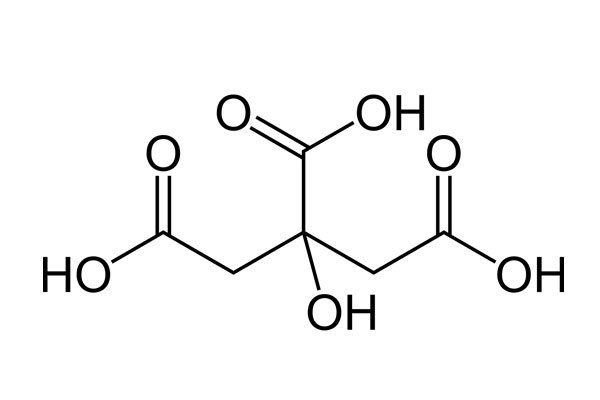
Chemical Formula: C6H8O7
Chemical Structure:
Physical Properties
General Properties: White powder, solid
Odor: Odorless
Intensity: 1,542 g/cm3
Boiling point: 310 °C
Melting point: 156 °C
Flash point: 155 °C
Vapor pressure:-
Refraction index: 1,493 nD
Solubility (aqueous) %59,2 w/w (20 °C)
General Properties
Citric acid, commonly known as sour salt, is a weak, organic and carboxylic acid. It is a hygroscopic chemical and it is found in both pure and hydrate forms. It is naturally found in animals and plants. Lemons can contain up to 8% citric acid. It is a commonly used acid and serves as pH stabilizer, sweetener and binder for ions or molecules. Citric acid, which is a monohydrate, can be made into dehydrated in 78°C.
Production
Citric acid is one of the most used acid. Although there have been many methods created since its discovery, fermentation is usually preferred in today’s chemistry.
Applications
Citric acid is frequently used, especially as food additive, as it is easily handled and relatively strong. It is also a clamping agent for metals. It is used for preventing lime in boilers and pipes. It also makes metals foam and perform better than cleaning products. Therefore, softening process is rendered unnecessary. Its 6% solutions are very effective for cleaning hard water tracks. It is also used as rust remover and it can pacify stainless steel.
It is also a budget-friendly pH stabilizer for cosmetic, dye, photography and many more businesses.
Safety Measures and Toxic Values
Although citric acid is a weak acid, an exposure to pure citric acid may cause negative effects. Constant exposure to its fume may lead to coughing, shortness of breath or sore throat. In high concentrations, it may cause vision loss. It may also cause rash if it contacts with skin. It may also damage tooth enamel if it is frequently consumed.