Cas No: 56-81-5
EINESC No: 100.000.263
Molecular weight: 92.094 gr/mol
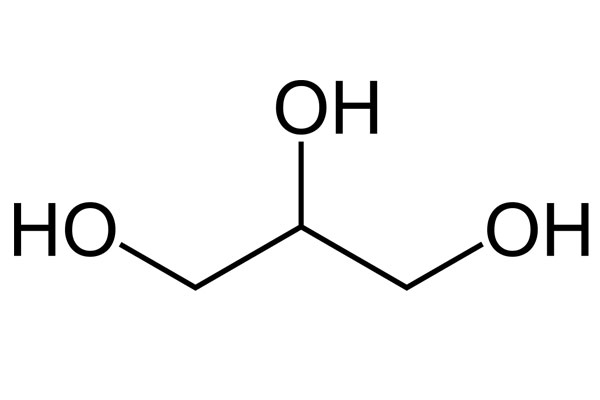
Chemical Formula: C3H8O3
Chemical Structure:
Physical Properties
General Properties: colorless, hygroscopic liquid
Odor: odorless
Intensity: 1,261 g/cm3
Boiling point: 290 °C
Melting point: 17,8 °C
Flash point: 160 ° C
Vapor pressure: 0,003 mmHg (50 ° C)
Refraction index: 1,4746 nD
Solubility (aqueous) completely miscible
Viscosity:
General Properties
Glycerin, or glycerol, is a colorless, odorless, sweet, viscose and non-toxic liquid. It can be produced both naturally and synthetically. It is an antiviral and antibacterial chemical. It has moisturising and sweetening effects.
Production
Glycerin is usually acquired by herbal and animal sources but it can be synthesized by petrochemistry, if needed.
Applications
Glycerin is used as hemuctant, solvent and sweetener in foods and beverages. It can be used as extender or thickener. It has less carbon hydrate and sweetness than sugar but it does not cause tooth decay.
It is frequently used in medical and cosmetic fields. It is an antiviral that prevents inflammation.
It is a good lubricant and it is used in toothpaste, shaving gels and soaps. It prevents skin dryness thanks to its hemuctant properties. It works as a cathartic when it is consumed orally.
Glycerin is also a frequently used solvent for extraction processes of plants. It is used as vaporizer electronic cigarettes.
Safety Measures and Toxic Values
Glycerin is not toxic in low doses. If it is consumed in high amounts, it may damage kidneys. It has low flammability. Nevertheless, it should not be mixed with strong oxidizers.