Cas No: 108-94-1
EINESC No: 203-631-1
Molecular weight: 98.15 gr/mol
Chemical Formuşla: C6H10O
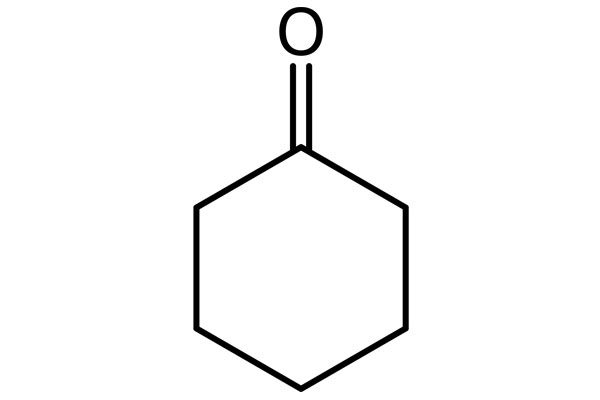
Chemical Structure:
Physical Properties
General Properties: luminous colorless liquid
Odor: pleasant, mint-like
Intensity: 0,9478 g/cm3
Boiling point: 155,65 °C
Melting point: −47 ° C
Flash point: 44 °C
Vapor pressure: 5 mmHg (20 °C)
Refraction index: 1.447 nD
Solubility : 8,6 g/100 mL (20 °C)
Viscosity:
General Properties
Cyclohexenone is a chemical compound that is formed by addition of ketone functional group to six carbon molecules which are in cyclic form. It has an odor similar to acetone and it is a colorless, lipoid liquid. It is partly mixed with water but it can be completely mixed with commonly used organic solvents. Cyclohexenone can take up yellow color. It is produced in high amounts as it is a pioneer substance for nylon production.
Production
Cyclohexnone is industrially produced by oxidation of cyclohexane and air which are catalyzed by cobalt. Cyclohexanol is also produced in this process and it, along with cyclohexane, is called KA OIL which means ketone-alcohol oil. This product is raw material for apidic acid.
Alternatively, it can be acquired from partial hydrogenetion of phenol. Studies are still conducted on newer methods.
Applications
95% of the produced cyclohexenone is used for nylon 6 and nylon 66 productions. Therefore, the above-mentioned KA oil is oxidised with nitric acid to create apicid acid. The produced acid is one of two raw materials of nylon 6. As for nylon 66, cyclohexenone is transformed into oxime which is needed to produce nylone 66.
It is also used in agricultural chemicals, glues, fuel and dye additive substances and it is also used as oil remover.
Safety Measures and Toxic Values
Cyclohexenone is extremely flammable and its fumes can create explosive mixtures with air, especially in temperatures above 44 degrees. It spreads to surface as it has more weight than air. Therefore, it must be stored in tightly closed container and in well-aired, cool environments.
It is not carciogenic for humans but it is mildly toxic. It is irritant for airways. It may cause serious injuries if it contacts with eyes and skin.