Cas No: 7697-37-2
EINESC No: 231-714-2
Molecular weight: 63.012 g/mol
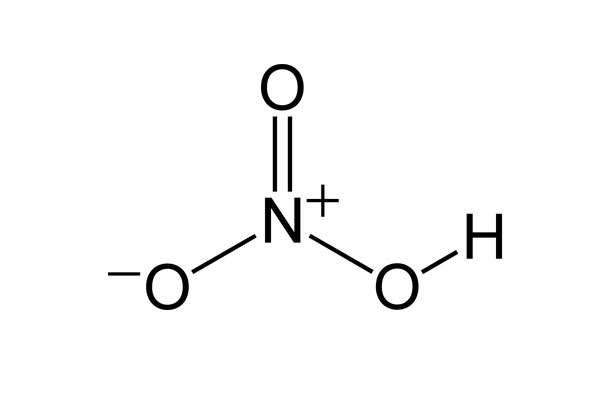
Chemical Formula: HNO3
Chemical Structure:
Physical Properties
General Properties: colorless, liquid which as red or yellow-ish fume
Odor: Strong, acetic
Intensity: 1.32 g/mL3
Boiling point: 103,4 °C
Melting point: −42 °C
Flash point:-
Vapor pressure: 48 mmHg (20°C)
Refraction index: 1,394 nD Solubility : Completely miscible
Çözünürlük(suda) : Tamamen karışabilir.
General Properties
Nitric acid, or commonly knwon as vitriol, is an extremely abrasive, inorganic mineral acid. It is one of the strongest acids and can easly be disintigrated. It is a very strong oxidizing agent. It forms its oxides with some metals and non-metals. When it combines with hydrochloric acid, it becomes the only compound that can dissolve gold. In its high concentrations, it creates a lot of smoke. Although it is colorless in pure form, it disintigrates into nitrogen oxide and water when steeped. Then,it can take on nitrogen oxide’s characteristic color, yellow.
Although it is sold as 68% in markets, it is possible to find higher concentrations. After 86% concentration, it is named as fuming nitric acid. It is called red fuming nitric acid up to 95% and white fuming nitric acid for 95% and beyond.
Production
Nitric acid is generally acquired from reaction of nitrogen dioxide(NO2) and water for trading purposes. The necessary nitrogen dioxide for this process is created by ostwalt method. In this process, waterless ammonia, in high temperature and under pressure, oxidized into nitrogen monoxide with the help of a catalyst such as platin. Then it is put in reaction with oxygen for creating nitrogen dioxide. Then it is dissolved in water to create nitric acid.
Besides this, countries like Norway, used Birkeland-Eyde method. It draw high amounts of energy from nitrogen gas in the air and produce nitric acid which can be used as renewable energy source.
Applications
Nıtrıc acid’s main production field is fertilizer manifacturing. More than 70% of its production is used for ammonium nitrate. It can also be used in nylon, plastic and polyurethane productions. It is one of the main substances to create adipic acid which is used in polymerizing reactions. It is also used in making explosives such as TNT. Nitric acid is also used in rocket fuels that go exoatmosphere.
Besides these, nitric acid is also used in cleaning products as an additive in lime remover. It is also used in metal industry.
Safety Measures and Toxic Levels
Nitric acid can be fatal if swallowed or inhaled. It may also cause permanent lung diseases like asthma. If it contacts with eyes, it may cause permanent blindness. If it contacts with skin, it may cause serious injuries or allergic reactions. It easily yields hydrolysis with proteins or fat and disintigrates living tissue. In case of any exposure, going out for a fresh air and consulting a doctor is advised. In case of skin contact, it must be sluiced for 10 to 15 minutes.
It may also cause fire or explosion when it goes in a reaction with easily oxidizable organic substances as it is a strong oxidizer. Therefore, it is advised to be cautious when mixing it with organic substances and alcali. It must be kept away from direct fire or high temperatures. It can also lose stability in high temperatures. It must not be provided to a city network as it is highly hazardous to environment.